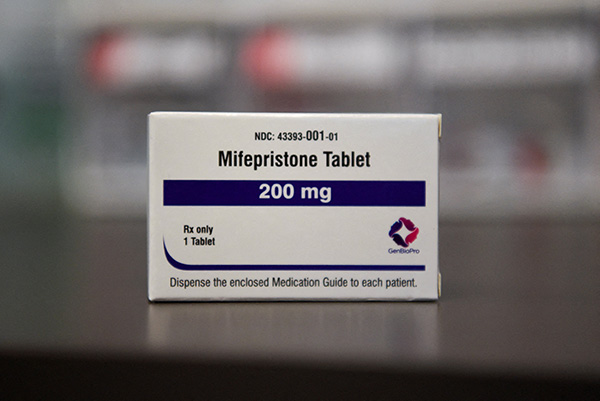
WASHINGTON — The Supreme Court said it will hear arguments on March 26 about how patients can access the commonly used abortion pill, mifepristone.
The Biden administration and the drug manufacturer are appealing a ruling from a federal appeals court that had significantly blocked access to this drug: banning the pills from being sent through the mail and shortening the window it can be used to terminate pregnancies from the current 10 weeks gestation to seven weeks.
An emergency ruling by the Supreme Court in April has kept access to mifepristone unchanged.
Earlier in April, U.S. District Judge Matthew Kacsmaryk in Texas sided with abortion opponents challenging the FDA’s initial approval of mifepristone in 2000 as a safe and effective medication and suspended its FDA approval. In response, the Justice Department filed an emergency appeal to the 5th Circuit.
The three-judge panel of the U.S. Court of Appeals for the Fifth Circuit said the drug’s initial approval should not be revoked, but they agreed with the Texas judge that the challengers were likely to win on other claims, such as the drug’s availability by mail and a provision that allowed the pills to be prescribed to women at up to 10 weeks of pregnancy.
The Supreme Court, in a brief order in late April, put the lower court rulings on hold pending appeals, leaving access to the abortion pill in place for now. It turned down the appeal regarding mifepristone’s safety and effectiveness.
The lawsuit against the abortion pill was filed last November by the group Alliance for Hippocratic Medicine on behalf of itself and member groups such as the Catholic Medical Association, the Christian Medical and Dental Associations, and other pro-life groups, represented by the religious liberty law firm, Alliance Defending Freedom.
Their lawsuit claimed the FDA “ignored the potential impacts of the hormone-blocking regimen on the developing bodies of adolescent girls” and disregarded evidence that chemical abortions cause more complications, particularly bleeding, than surgical abortions.
Mifepristone, also known as RU-486, is the first of a two-drug regimen used to end a pregnancy in its early stages.
Leaders of the U.S. Conference of Catholic Bishops have been vocal in their opposition to the drug since it was first given FDA approval. They echoed objections in 2016 when the FDA relaxed rules for its use, saying it could be administered with fewer visits to a doctor, and they also objected earlier this year when the FDA announced it was allowing some retail pharmacies to distribute the drug.
